Balancing Cost, Safety, and Performance for a Sustainable Future
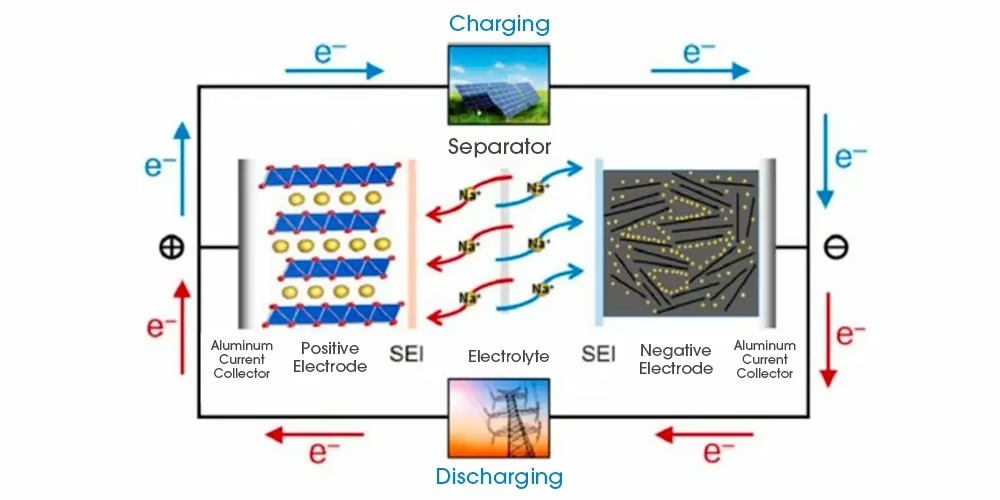
Sodium-ion batteries represent a type of rechargeable battery that operates by shuttling sodium ions between the positive and negative electrodes—functioning in a manner similar to lithium-ion batteries.
Owing to this promising technology, the International Union of Pure and Applied Chemistry (IUPAC) recognized sodium-ion batteries as one of the Top Ten Emerging Technologies in Chemistry in 2022.
How Sodium-Ion Batteries Work
During charging and discharging, sodium ions (Na⁺) move back and forth between the two electrodes. While charging, they leave the cathode, travel through the electrolyte, and embed into the anode. During discharge, this process reverses, and the ions return to the cathode.
For example, the new 18650-format sodium-ion battery specifically uses the movement of sodium ions—rather than lithium ions—to store and release electrical energy.
Key Technical Advantages
Researchers have classified certain materials used in these batteries as trade secrets. As Dr. Loïc Simonin, a collaborating scientist at LITEN, points out: “Their energy density is now competitive with that of lithium iron phosphate (LiFePO₄) batteries.”
Sodium-ion batteries primarily use sodium-based electrode materials. Compared to lithium salts, sodium salts are far more abundant and cost significantly less. Because sodium ions are larger than lithium ions, these batteries offer an economical alternative where high energy density or low weight is not the main priority.
Compared to lithium-ion batteries, sodium-ion technology brings several clear benefits:
Lower material cost: Sodium sources are widely available and inexpensive. Using iron-manganese-nickel-based cathode materials can cut raw material costs by nearly half compared to the ternary cathodes used in lithium-ion batteries.
Improved electrolyte efficiency: Thanks to the properties of sodium salts, these batteries perform well with low-concentration electrolytes. At equal concentrations, sodium electrolytes show about 20% higher conductivity than their lithium counterparts, which helps reduce costs.
Lighter and cheaper current collectors: Since sodium does not form alloys with aluminum, manufacturers can use aluminum foil (instead of copper) as the anode current collector. This cuts costs by about 8% and reduces weight by roughly 10%.
Zero-volt discharge capability: Sodium-ion cells can safely discharge to zero volts, a feature lithium-ion batteries lack. With energy density exceeding 100 Wh/kg—comparable to lithium iron phosphate batteries—sodium-ion systems offer clear cost advantages, making them strong candidates to replace lead-acid batteries in large-scale energy storage.
Role in the Energy Storage Market
With clear strengths in low-temperature performance, safety, and cost-effectiveness, sodium-ion batteries are set to become an important supplement to the energy storage market.
They are particularly well-suited for grid-side storage, user-side storage, and renewable integration (such as solar and wind), where they help reduce the levelized cost of storage and enhance system stability.
Industry analysts project that by 2030, sodium-ion batteries will account for about 10% of the global energy storage battery market.
In a sign of this transition, the company LVFU has announced that it will begin using sodium-ion batteries in projects starting this December.

